When your prescription switches from a brand-name pill to a generic one, it’s not just a cost-saving move-it’s a decision that affects your body directly. You might not think much of it. After all, the label says the same active ingredient. But what happens when your body reacts differently? Clinical studies show the answer isn’t as simple as ‘they’re the same.’ For most people, switching to generics is safe and effective. For others, especially those on medications with tight therapeutic windows, the switch can trigger real, measurable risks.
What Does ‘Bioequivalent’ Really Mean?
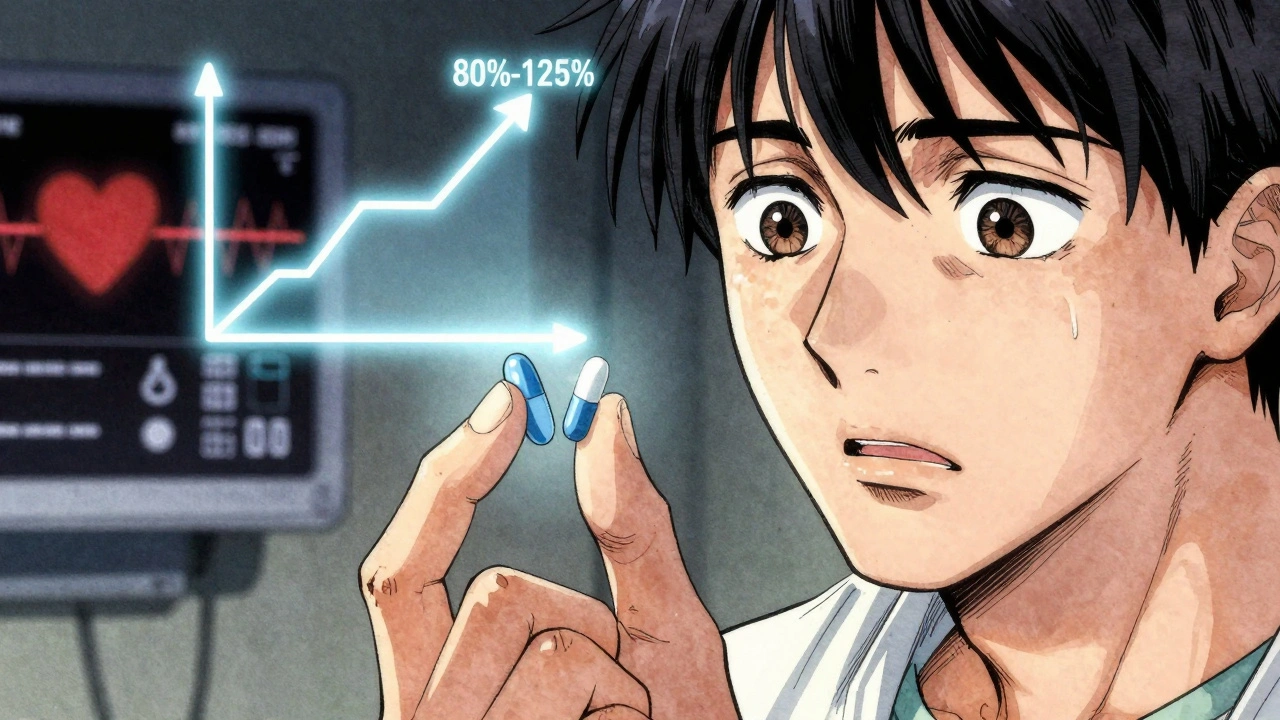
The U.S. Food and Drug Administration (FDA) requires generic drugs to be bioequivalent to their brand-name counterparts. That means the generic must deliver the same amount of active ingredient into your bloodstream within the same timeframe. The acceptable range? Between 80% and 125% of the brand’s absorption rate. At first glance, that sounds precise. But 20% variability? For drugs where even a 5% change in blood levels can mean the difference between control and crisis, that’s not just a technicality-it’s a clinical concern.Take levetiracetam, a common antiepileptic drug. A study of 760 patients found that after switching to generic versions, nearly one in five reported new or worsening side effects: blurred vision, headaches, memory loss, mood swings, even depression. Some patients who were seizure-free for years suddenly had breakthrough seizures. Blood tests showed their drug levels dropped by 22% to 31% compared to when they were on the brand-name version. These aren’t anecdotes. These are measurable changes tracked in controlled clinical settings.
When Generics Work Better-And When They Don’t
It’s easy to paint generics as universally good or bad. The data doesn’t support that. For many medications, especially those used to treat high blood pressure or high cholesterol, generics outperform brands-not just in cost, but in outcomes.A massive 2020 study across Austria’s entire insured population (8.5 million people) found that after adjusting for age, health status, and other factors, patients on generic statins like simvastatin and atorvastatin had fewer heart attacks, strokes, and deaths than those on the brand-name versions. The same study showed that patients were more likely to keep taking their generic blood pressure meds than their branded ones. Why? Probably because they cost less. Better adherence means better control.
But not all drugs follow that pattern. For bisoprolol and nebivolol, two beta-blockers used for heart conditions, the data flipped. Patients on generics had worse outcomes. Another study tracking 88,600 patients switching from brand to generic blood pressure drugs found a 5.4% spike in emergency room visits within six months. These aren’t errors in the data-they’re signals that some drugs behave differently once the brand name is gone.
The Hidden Cost of Switching: Adherence, Confusion, and Duplication
The problem isn’t just about how the drug works in your body. It’s about how you understand it.A 2023 study of 218 patients found that only 43 of them-about 20%-could correctly say what their medication was for. Most identified their pills by color, shape, or markings. When a generic changes its appearance-say, from a blue oval to a white capsule-patients think they’ve been given the wrong medicine. They stop taking it. Or worse, they double up, thinking they missed a dose. One in ten patients in that study accidentally took the same drug twice because they didn’t recognize the new generic version.
Some patients switched between five different generic manufacturers over five years. That’s not a typo. Five different versions of the same drug. Each with a different look, different fillers, different release profiles. And each time, the patient had to relearn what they were taking. No wonder adherence drops.

Who’s at Risk? The Real Red Flags
Not everyone needs to worry. But certain groups are more vulnerable:- People with epilepsy-especially those with unstable seizures or on multiple antiepileptic drugs.
- Patients on narrow therapeutic index (NTI) drugs-like warfarin, lithium, phenytoin, or cyclosporine-where tiny changes in blood levels can cause toxicity or treatment failure.
- Older adults with multiple prescriptions, where pill appearance changes trigger confusion.
- Patients with mental health conditions-switching generics for antidepressants or antipsychotics has been linked to relapse in some cases, even if not always statistically significant.
Doctors don’t always warn patients about this. A 2012 statement from the American Academy of Neurology says it clearly: ‘Some patients may experience problems with generic substitution.’ They recommend monitoring drug levels and being ready to switch back if symptoms appear.
What Does the Law Say? It Depends Where You Live
In the U.S., pharmacists can automatically substitute a generic unless the doctor writes ‘dispense as written’ or the patient refuses. Forty-nine states allow this. In the European Union? No automatic substitution. The doctor must specifically authorize the switch. Canada and Australia follow similar rules-prescriber consent is required for NTI drugs.This isn’t just bureaucracy. It’s safety. In places where pharmacists can switch without input, studies show higher rates of patient confusion and discontinuation. In places where doctors control the switch, outcomes are more stable.

What Should You Do?
If you’re on a medication with a narrow therapeutic index-especially antiepileptics, blood thinners, or heart drugs-don’t assume a generic is automatically safe. Here’s what to do:- Ask your doctor: Is this drug on the list of those where switching has shown risks? Ask specifically about phenytoin, levetiracetam, carbamazepine, warfarin, or lithium.
- Check your pill: If the shape, color, or imprint changes, ask why. Don’t assume it’s the same drug.
- Request therapeutic drug monitoring: If you’re switching, ask for a blood test before and after to confirm levels haven’t dropped.
- Track symptoms: Keep a journal. Note any new headaches, dizziness, mood changes, or seizures. Even small shifts matter.
- Speak up if something feels off: If you feel worse after a switch, don’t wait. Contact your doctor. You’re not imagining it.
For most people taking common meds like metformin, lisinopril, or atorvastatin, generics are a win. They save money. They work. But for others, the switch isn’t just a cost-cutting measure-it’s a clinical event that needs oversight.
The Bigger Picture: Cost vs. Control
Generics save the U.S. healthcare system $370 billion a year. That’s real money. But that savings can vanish fast if a patient has a seizure, ends up in the ER, or has a heart attack because their drug levels dropped. One study found the average cost of managing a breakthrough seizure after switching to a generic antiepileptic was $1,850 per incident. Multiply that by thousands of patients, and you’re not saving money-you’re just shifting the cost.The goal isn’t to stop generics. It’s to make switching smarter. Better tracking. Better communication. Better rules for high-risk drugs. The FDA is already moving in that direction-drafting new guidelines for bioequivalence testing of complex generics, especially antiepileptics. The European Medicines Agency now advises extra caution for patients with unstable epilepsy or liver problems.
Science doesn’t say generics are bad. It says: context matters. The same pill can be safe for one person and risky for another. That’s not a flaw in the system. It’s a reminder that medicine isn’t one-size-fits-all.
Are generic drugs always as effective as brand-name drugs?
For most medications, yes. But not always. For drugs with narrow therapeutic indices-like antiepileptics, blood thinners, or immunosuppressants-small differences in how the body absorbs the drug can lead to treatment failure or side effects. Clinical studies show some patients experience breakthrough seizures, unstable blood pressure, or higher hospitalization rates after switching to generics. The FDA’s bioequivalence standard allows up to 20% variability in absorption, which is fine for many drugs but risky for others.
Why do some patients feel worse after switching to a generic?
It’s often not about the active ingredient-it’s about the fillers, coatings, or release mechanisms. These non-active components can affect how quickly the drug dissolves in your body. For people with sensitive conditions like epilepsy or heart failure, even a slight delay or spike in absorption can trigger symptoms. Some patients also experience psychological effects, thinking the generic is ‘weaker’ because it looks different, which can worsen perceived side effects.
Can pharmacists switch my brand-name drug to a generic without telling me?
In most U.S. states, yes. Pharmacists can substitute a generic unless your doctor writes ‘dispense as written’ or you specifically opt out. In Australia, Canada, and most of Europe, the prescriber must approve the switch, especially for high-risk drugs. Always ask your pharmacist if a substitution was made, and check your pill’s appearance each time you refill.
Should I ask my doctor to keep me on the brand-name drug?
If you’re on a drug with a narrow therapeutic index-like levetiracetam, phenytoin, warfarin, or lithium-and you’ve been stable on the brand, yes. Ask your doctor if switching is necessary. If you’ve had side effects or loss of control after a switch, document it and request the brand. Insurance may require prior authorization, but your medical history supports your case.
How do I know if my generic is causing problems?
Watch for changes: new or worsening side effects, loss of symptom control, unusual fatigue, dizziness, or mood shifts. If you’re on an antiepileptic and have a breakthrough seizure, or on a blood thinner and your INR fluctuates, that’s a red flag. Keep a symptom log and bring it to your doctor. Ask for a blood test to check drug levels. Don’t ignore small changes-they can be early warnings.
Adam Everitt
December 11, 2025 AT 15:04so like... generics are just brand names with a bad haircut? i mean, same active stuff right? but why does my head feel like it’s been stuffed with wet cotton since they switched me? maybe it’s the fillers. or maybe i’m just imagining it. either way, my neurologist shrugged. so here i am, confused and slightly dizzy.
Nathan Fatal
December 12, 2025 AT 21:24The FDA’s 80–125% bioequivalence window is a regulatory loophole disguised as science. For NTI drugs like warfarin or phenytoin, that 45% swing isn’t ‘acceptable’-it’s a gamble with your life. Real-world data confirms this: seizure spikes, INR chaos, ER visits. The system isn’t broken-it was designed this way to save pennies, not lives. We need mandatory therapeutic drug monitoring for high-risk meds, period. No more automatic substitutions without prescriber consent. This isn’t about brand loyalty. It’s about pharmacokinetic integrity.
Laura Weemering
December 13, 2025 AT 17:55Okay, but… have you considered that maybe… it’s not the drug? Maybe it’s the *fear*? The placebo/nocebo effect is terrifyingly powerful. People see a different pill, panic, and their bodies *respond*. Studies show that even when patients are told they’re on the exact same drug, if it looks different, they report more side effects. And don’t get me started on the ‘I’m not getting the same medicine’ narrative-it’s not science, it’s trauma. Also, why do we assume every patient is capable of tracking INR levels or remembering pill colors? We’re not all pharmacists. The system needs to protect the vulnerable, not burden them with vigilance.
Audrey Crothers
December 14, 2025 AT 18:21I switched my mom from brand to generic levetiracetam and she had a seizure two weeks later. I cried for hours. The pharmacist didn’t even tell me they switched it. I found out because the pill looked different. I called the doctor. They said ‘it’s the same.’ But it wasn’t. My mom’s brain knew the difference. Don’t let them tell you it’s all in your head. If you feel worse after a switch-trust yourself. Fight for your brand. Your life matters more than the $20 savings.
Stacy Foster
December 15, 2025 AT 13:21THE PHARMA COMPANIES ARE LYING. THEY PAID THE FDA TO LET THEM SLIP IN THESE FAKE GENERIC VERSIONS. THEY WANT YOU TO GET SEIZURES SO YOU’LL GO BACK TO THE BRAND AND PAY MORE. THIS IS A SCAM. THEY’RE USING FILLERS TO MAKE YOU DEPRESSED SO YOU’LL BUY MORE DRUGS. I SAW A VIDEO ON TIKTOK WHERE A WOMAN’S BRAIN WAS ‘REPROGRAMMED’ BY THE COATING ON HER GENERIC PILLS. THEY’RE TESTING ON US. STOP TRUSTING THE SYSTEM. CHECK YOUR PILLS. TAKE PHOTOS. FILE COMPLAINTS. THEY’RE WATCHING.
Reshma Sinha
December 17, 2025 AT 03:00As a clinical pharmacist in Mumbai, I’ve seen this firsthand. In India, generics dominate-but we have a culture of patient education. Pharmacists sit down, show the patient the pill, explain the switch, and ask for feedback. No automatic substitution. No surprise changes. We track adherence via SMS reminders and pill-count audits. Outcome? Lower relapse rates than in the U.S. It’s not about the drug. It’s about the system. We need empathy, not just bioequivalence. Human connection > regulatory thresholds.
Ashley Skipp
December 17, 2025 AT 09:02People are so dramatic. If your meds aren't working you're probably just lazy or noncompliant. Generic is generic. Same chemical. End of story. Stop making excuses. Your doctor knows best. If you can't afford the brand tough luck. Your life isn't special enough to warrant special treatment.
nikki yamashita
December 19, 2025 AT 03:40My husband’s on warfarin. We always ask for the brand. Insurance fought us. We won. No regrets. If you’re stable, don’t risk it. Your body remembers. And if you feel weird? Say something. Always. Seriously. It’s not drama. It’s survival.